Invitado Internacional: Tratamiento de Hepatitis B
Hepatitis B Treatment: new directions for future therapy
María H.Sjögren, M.D., Mph, Clinical Gastroenterologist, Department Of Clinical Investigation,
Walter Reed Army Medical Center, Washington D.C. Tomado De Resúmenes De Congreso De
Actualización En Gastroenterología, Celebrado Entre El 23 -25 De Marzo De 2001 En El Walter
Reed Army Medical Center, Washington D.C., Con Autorización Del Autor. Traducción: Luis
Alejandro Orozco, Gastroenterólogo, Endoscopista, Clínica Pastrana, Iss, Bogotá, D.C.
Introducción
Chronic infection with the hepatitis B virus (HBV) is a common occurrence in the world. Worldwide, approximately 400 million subjects were HBV-infected by the year 2000, with 1.25 million of them located in the United States (1). In the USA, HBV infection is responsible for 5 to 10% of all causes of chronic liver disease and approximately 200 cases of fulminant hepatitis per year. HBV causes the major morbidity and mortality when it progresses to cirrhosis and hepatocellular carcinoma.
The goal of therapy is to halt the progression of liver injury by suppressing viral replication and eliminating infection. When a sustained loss of HBeAg and HBV DNA is achieved, a biochemical, clinical and histological remission ensues. The HBV precore mutant deserves particular mention. The HBV precore mutant is devoid of the capacity to generate HBeAg; therefore infected subjects are HBeAg and anti-HBe negative but still are HBV DNA-positive. In these cases the goal of therapy is to eliminate viral replication and induce a HBV DNA-negative status.
All HBV-infected subjects with active viral replication should be treated unless there are contraindications for therapy. Even if there is minimal evidence of hepatic inflammation, the presence of persistent viremia foretells liver disease sometime in the future.
The indications for therapy are (2):
- Detectable HBeAg and HBV DNA for at least 6 months
- Increased serum ALT level
- Liver biopsy showing chronic inflammation
Liver biopsy is not an absolute requirement,but may be useful. Once therapy starts,the patients need to be monitored at monthly visits where HBV DNA, HBeAg,anti-HBe and serum ALT status are evaluated.
The preferred method to test for HBV DNA during therapy is the liquid hybridization assay. The polymerase chain reaction (PCR) assays are uniquely sensitive and may detect HBV even in subjects who have a complete clinical remission of HBV infection.
Medications for Treatment of Chronic HBV Infection (3)
- Interferon alpha
- Antiviral agents or nucleoside analogues
- Immunomodulator therapy
Non-HBV specific
Thymosin
Interleukin-2
Interleukin-12
Levamisole
HBV-specific
Pre-S or S peptide vaccination
Cytotoxic lymphocyte epitope vaccination
DNA vaccination
Interferon a
Interferon alfa-2b was approved in 1992 for the treatment of chronic HBV infection. The recommended doses are 5 million units (MU)injected subcutaneously (SC) every day for 16 weeks or 10 MU injected SC three times a week for 16 weeks. In two-thirds of treated subjects a transient flare of ALT is observed usually between weeks 4 -12 of therapy or after therapy is discontinued. This flare is believed to be a result of immunemediated clearance of HBV-infected hepatocytes. Interferon has been successful in about one third of treated patients. The addition of a 6-week “priming ” of prednisone before starting interferon did not result in a higher remission rate (4). The use of prednisone in the treatment of HBV is discouraged.
A meta-analysis of 16 randomized clinical trials (5) have shown that 33% of treated subjects lose HBeAg and 32% lose HBV DNA.These rates compared favorably to controls, which showed 12 and 17% loss of these virological markers (p<0.001). In general, the sustained response following interferon therapy is 30 to 40%.
Table summarizes results of reference 4.
Long-term benefit (prevention of cirrhosis or increased survival)has not been adequately assessed. A limited study of 103 interferon-treated patients showed a 95% 5 year survival rate in subjects who had a sustained remission, and less than 50%among those who did not (6).
Side effects of interferon therapy are well known, among them are fever, malaise, myalgia, granulocytopenia and thrombocytopenia. In less than 5%, thyroid suppression or significant psychiatric side effects may be observed. If the side effects are serious enough, interferon doses are modified or in rare situations, discontinued permanently.
Interferon therapy can be given to cirrhotic patients, but with caution and frequent follow-up because it may lead to decompensation due to exacerbation of HBV.
Factors that predict a favorable response to interferon therapy are (3):
- HBV DNA <100 IU/ml)
- High levels of serum ALT (>100 IU/ml)
- Evidence of necroinflammatory process in the liver
Other favorable factors are: female gender, absence of immunosuppression, short duration of HBV infection, wild-type (HBeAg-positive) infection, horizontal transmission.
Nucleoside Analogues (3)
First generation. Vidarabine, Acyclovir, Gancyclovir, Zidovudine, Ribavirin, Didanosine, Zalcitabine.
Second generation. Fialuridine (FIAU), Lamivudine, Famciclovir, Lobucavir, Adefovir (nucleotide analogue), BMS 200475, Emtricitabine.
The first generation agents were not successful in treating chronic HBV. Among the second generation, FIAU caused severe mitochondrial dysfunction in subjects treated for more than two months (7).
Lamivudine
Licensed first for the treatment of HIV, in 1998 was approved for the treatment of chronic HBV in the USA. Glaxo Wellcome manufactures it as Epivir®, an oral preparation. It is recommended as a single 100 mg daily dose for at least 1 year. However, it seems reasonable to continue treatment until HBeAg seroconversion is documented. Lamivudine decreases HBV replication by competitively inhibiting viral reverse transcriptase and terminating proviral DNA chain extension.
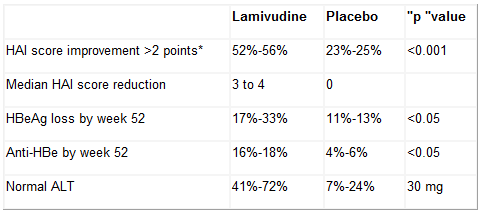
Findings in clinical trials of treatment-naive and non-responders to interferon findings are summarized in the following table (8, 9):
HAI = Histologic Activity Index (assesses periportal and bridging necrosis, intralobular degeneration and focal necrosis, portal inflammation, and fibrosis or cirrhosis).
In addition, clinical trials in HBV-infected Asian subjects and patients who are infected with the HBV precore mutant have responded in a similar manner described in the above table (10). These populations have not responded in a less successful manner to interferon treatment.
(Lea También: Tratamiento de Hepatitis B)
Lamivudine YMDD Mutant
The YMDD motif is a highly conserved domain of reverse transcriptases and is required for polymerase activation. Long term lamivudine treatment induces resistance to the drug due to mutation at the YMDD locus. The rate of YMDD mutations ranges between 16 to 32% after 1 year of treatment with lamivudine, increases to 40% after 2 years and 50% after three years. Several mutations have been characterized, but the better described are the substitution of valine or isoleucine for methionine at residue 552 (M552V) and the substitution of methionine for leucine at position 528. Other mutations at residues 501 and 515 exist but their clinical significance is unknown.Mutations of the YVDD and YIDD locus have also been recorded (11-12).
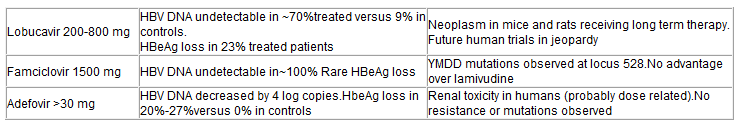
Status of Other Nucleoside Analogues
Immunomodulator and Combination Therapy
Treatment with thymosin for 6 to 12 months was associated with increased sustained response in some trials (13-14), but the results were not confirmed by other studies (15-16). Mutation of HBV has been reported after treatment with thymosin (17). Other immunomodulators such as therapeutic vaccines for HBV infection have shown initial promise; clinical trials are necessary to evaluate the benefit of these therapies.
The combination of interferon and nucleoside analogues has been tried with conflicting results. It is likely that other regimens including immunomodulator therapy and nucleoside analogues may yield an effective response. The new pegylated interferons may also allow new approaches to the therapy of chronic HBV.
Therapy of Hepatitis B after liver Transplantation
HBV disease accounts for less than10% of all liver transplantations in the USA. Prior to therapy with HBIG and lamivudine, recurrence of HBV was observed in more than 90% of the newly transplanted livers. With the dual medication regime, the recurrence rates have dropped to less than 10%, making return of HBV a rare clinical problem. However, there are patients who either acquire HBV after transplantation or “breakthrough “while receiving dual therapy with HBIG and lamivudine. In the first case, treatment with lamivudine is indicated, alone or in combination with HBIG. Because of immunosuppression, resistance to lamivudine should be expected in approximately 50% of the cases. In the “breakthrough “cases, use of adefovir should be considered.
Conclusión
Chronic hepatitis B needs to be treated to avoid the morbidity and mortality associated with the late stages of the disease. Some experts in the field have advocated the use of interferon in patients who have a good likelihood of response (non-cirrhotic, HbeAg positive, horizontally infected, with abnormal ALT and low HBV DNA levels). If the patient does not seroconvert to anti-HBe, lamivudine could be used at a later time.
Lamivudine is reser ved as initial therapy for patients who do not have the characteristics that were described as favorable for interferon therapy, particularly for subjects infected with the HBV precore mutant or who were infected at the time of birth. Lamivudine may have to be continued indefinitely in patients who do not seroconvert because of the risk of decompensation if therapy is stopped while the virus is still replicating.

CLIC AQUÍ Y DÉJANOS TU COMENTARIO